Access criteria policy
This policy sets out the scope and principles for how Pharmac develops access criteria for funded pharmaceuticals.
Context
Access criteria are the criteria that form a restriction applied to a Pharmaceutical Schedule (the Schedule) listing. At present, these criteria are found mainly in Special Authorities which are a type of restriction where an approval is necessary for the patient to receive the funded treatment.
The purpose of access criteria when applied by Pharmac to funded pharmaceuticals is to effectively target funding to the specific population that is likely to gain the most benefit.
This targeting is needed to manage:
- Financial scarcity – targeting allows funding of a medicine, to any extent, within a financial constraint
- Resource scarcity – targeting ensures that products are available when they are needed, now and into the future
- Ensuring that Pharmac achieves its statutory objective of achieving best health outcomes within a fixed budget.
The principles in this policy are intended to allow access beyond a tightly targeted population where necessary, so that people with inequitable access and poor health outcomes do not miss out on funded treatment. Access criteria should account for challenges faced by any population group experiencing a disparity of access and/or health outcome.
In some cases, this may mean that the boundary line for funded treatment is drawn to include other people, as in the diagram below.
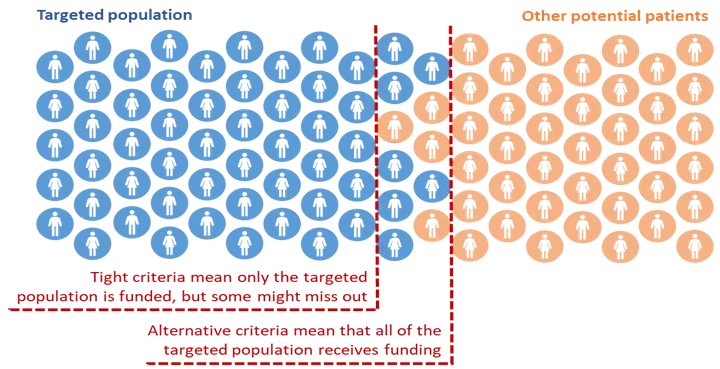
This assumes that the population and range of contexts for treatment will be clearly described before identifying the access requirements that are the best fit in each case.
This policy is supported by a set of Pharmaceutical Schedule Standards. These standards are guidance documents, providing a position or clarity on the application of features of this policy.
Scope
This policy applies to all Pharmac staff when:
- developing, reviewing and amending Schedule access criteria
- seeking and applying expert advice and supplier guidance in the development and amendment of Schedule access criteria
- developing procedures, or any other guiding documentation for the construction of Schedule rules that include access criteria.
The scope of the Schedule access criteria policy includes criteria embedded within rules for pharmaceuticals that are funded, or under consideration for funding, with restrictions (such as a Special Authority) in community and hospital settings.
Principles
Pharmac staff will act in accordance with the following principles within processes that consider new funding applications, changes to existing access or reviews of access criteria.
We will clearly identify the target population for funded treatment
- A decision in principle will be recorded to fund a treatment for a specified target population.
- We will define the target population by clinical condition (indication). People with the same clinical condition should be identified and described similarly in terms of target population.
- We will not define the target population by demographics such as age or ethnicity.
- We will not limit the target population to provision by a specific service or provider type.
Access criteria will practically describe the identified population, to the extent possible
- Access criteria will be developed consistent with this policy and standards, based on clinical condition (indication) and associated clinical criteria.
- People with the same indication will not be differentiated by the cause of the same indication as a means of excluding access.
- People with the same indication but with different clinical situations or circumstances may be differentiated by access criteria.
- The intent of each access criterion will be clear, and we will avoid using time-based and aged based criteria as a proxy for clarity.
- Access criteria should be enduring, where possible.
- Access criteria should not be lifted directly from clinical trials/supplier provided criteria without understanding the intent of each criterion and relevance in practice to treatment in the NZ population.
- We should use access criteria in a way that removes barriers for everyone. Using access criteria in this way prevents the use of override criteria including demographics.
We will favour broader access over more restrictive access
- There may be physical, demographic, geographic or cultural challenges to meeting the requirements of access criteria that reflect the target population.
- Access criteria should enable the entire target population to access funded treatment.
- The boundary for inclusion may be drawn more widely than for an ‘ideal’ population, meaning that inclusion of other people may be needed to ensure full access by the target population.
- We will not rely on exceptions processes for edge-cases knowing such exception schemes are underutilised
- Financial risks will not be managed using access criteria. These should be solved commercially, to the extent possible.
Access criteria have a specific and limited purpose
- Access criteria will be applied in the context of managing limited funding and scarcity of resources.
- We will accommodate the potential for diverse patient journeys in access criteria.
- We will not use access criteria to manage safety or other prescribing issues, unless this can be justified as promoting responsible use in a way that is not open to other entities.
We will ensure each access criterion serves a specific, justifiable purpose in ensuring practical application of these principles
- Each access criterion for inclusion will be justified in relation to these principles and the Pharmaceutical Schedule Standards
- Ongoing retention of access criteria will be justified in relation to these principles and the Pharmaceutical Schedule Standards
Related documents
There are several policies and other documentation that support and supplement this policy. These include related policies, procedures, guidelines and standards that are still under development and will be linked to in due course.
Review and publication
This policy will be considered for review every five years, or more frequently, as needed. Review will follow Pharmac’s standard process for the review and development of organisational policy.
- Pharmac Access Criteria Policy December 2025 [PDF 183 KB]


