The future of managing hospital medical devices
The Government has clarified responsibilities for the procurement of medical devices to create efficiencies and avoid duplication.
On this page
New approach announced September 2025
Health NZ and Pharmac will each take responsibility for the procurement of medical devices that are most focused on their particular capabilities and expertise.
Pharmac worked together with Health NZ to identify which of the 55 categories of medical devices they should each be responsible for.
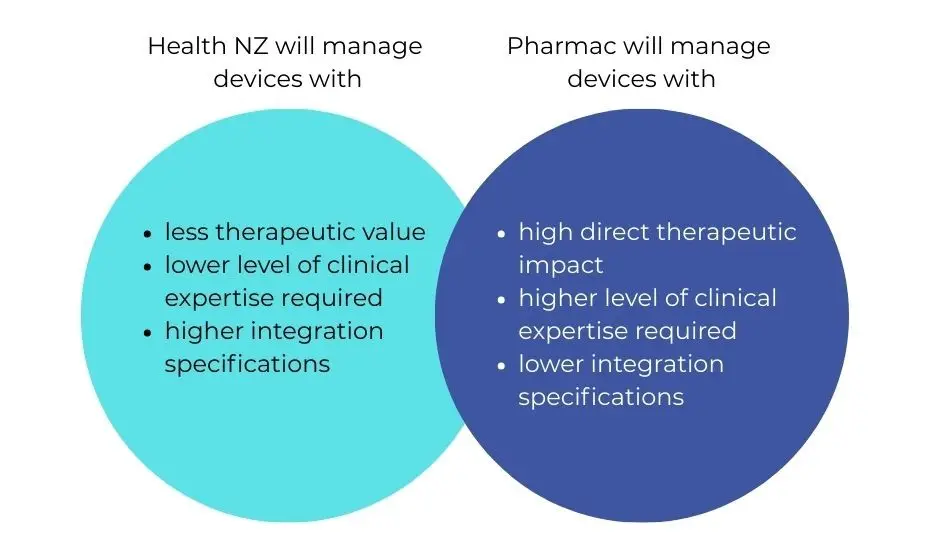
Pharmac will lead procurement for 27 categories, while Health NZ will lead 28. Pharmac will generally be the lead agency for devices that primarily have a direct therapeutic impact on patients, for example orthopaedic implants.
Health NZ will generally be the lead agency for devices that are less therapeutically intensive and require integration with infrastructure, for example, beds, imaging and diagnostics.
Health NZ will continue to manage the overall medical devices budget.
Pharmac will also focus on delivering high-quality, independent Health Technology Assessments to support access to new and innovative devices.
Why is this happening?
It makes sense for Pharmac and Health NZ to be able to procure medical devices that are most focused on their particular capabilities and expertise. A clear split between the agencies will provide the transparency and certainty that suppliers have been asking for.
When will this happen?
We are still working through the detail for this transition with Health NZ. We will add more details as they are available.
What medical devices will Pharmac be responsible for?
Pharmac will generally be the lead agency for devices that have a direct therapeutic impact on patients and that need a high level of clinical input. For instance, this includes orthopaedic implants.
Now that the Government has clarified responsibilities for the procurement of medical devices, we are developing the next level of detail, including how this split may affect current contractual arrangements and commercial processes underway. This detail will be shared as soon as it is available.
What medical devices will Health NZ be responsible for?
Health NZ will generally be the lead agency for devices that are less therapeutically intensive and require integration with infrastructure, for example, beds, imaging and diagnostics.
How Health Technology Assessments fit into this approach
Pharmac will deliver independent Health Technology Assessment advice to Health NZ.
Pharmac independently evaluates the clinical benefit and cost-effectiveness for a range of medical devices, particularly where these are highly technical. Pharmac assessments bring together clinical effectiveness, patient voice, cost utility, budget impact, implementation, commercial considerations and equity impacts.
This involves analysis and comparison of different technologies to inform Health NZ’s adoption of new devices and technologies and will eventually ensure that the most effective and efficient options are selected for use.
I have an existing contract with Pharmac, but my category is moving to Health NZ. What do I do?
Now that the Government has clarified responsibilities for the procurement of medical devices, we are developing the next level of detail, including how this split may affect current contractual arrangements and commercial processes underway. This detail will be shared as soon as it is available.
We’re working closely with Health NZ to understand what the changes mean for current agreements and procurement processes already underway, so we can plan for any transition.
In the meantime, your current contracts with Pharmac remain in place, and we’ll keep you informed of any updates as the process moves forward.
What happens if I’m currently in a procurement process?
We understand that suppliers and stakeholders will have questions about how this change may affect current and future contracts. At this stage, any current agreements with Pharmac remain in effect and we encourage you to continue with business as usual.
We are working closely with Health NZ to understand the implications for individual agreements and procurement processes. We will be in touch with further information as soon as we are able.
If you have any questions or would like to discuss your specific circumstances, email devices@pharmac.govt.nz or your contract manager.


