Zinc (Zincaps) 50 mg capsule: Supply issue
There is a supply issue affecting Zincaps (Pharmacode: 258253).
26 February 2026 | Resupply delayed
We understand that some pharmacies had difficulty accessing the Rugby brand zinc capsules. A shipment has now been released and stock should be available for pharmacies to order, however stock is low.
Unfortunately, the resupply of NZ-registered Zincaps has been delayed until April 2026. Pharmac is working to minimise any disruption to people who need zinc capsules.
Affected product
There have been delays at the factory making Zincaps. The following product has been unavailable since mid-October 2025.
- Chemical: Zinc sulphate
- Presentation: Cap 137.4 mg (50 mg elemental)
- Brand: Zincaps
- Pharmacode: 258253
- Subsidy: $11.00
- Measure / Qty: per 100
Pharmac is working with individual pharmacies to ensure supply is available for people with rare disorders.
Alternative products
Rugby Zinc
Rugby Zinc was funded from 1 January 2026. This is a product labelled for sale in the United States as a dietary supplement, rather than a prescription medicine. As such, there is no data sheet or product leaflet available.
- Chemical: Zinc sulfate heptahydrate
- Presentation: cap 220 mg (equivalent to 50 mg elemental zinc)
- Pharmacode: 2719665
- Subsidy: $29.14
- Measure/Qty: per 100
- s29 and wastage claimable
Key differences:
- The Rugby capsule size is 220 mg compared to Zincaps 137.4 mg (zinc content is still the same = 50 mg).
- Chemical name is zinc sulfate heptahydrate, instead of zinc sulphate monohydrate.
- Despite this product being labelled "dietary supplement", it is still a prescription medicine in New Zealand.
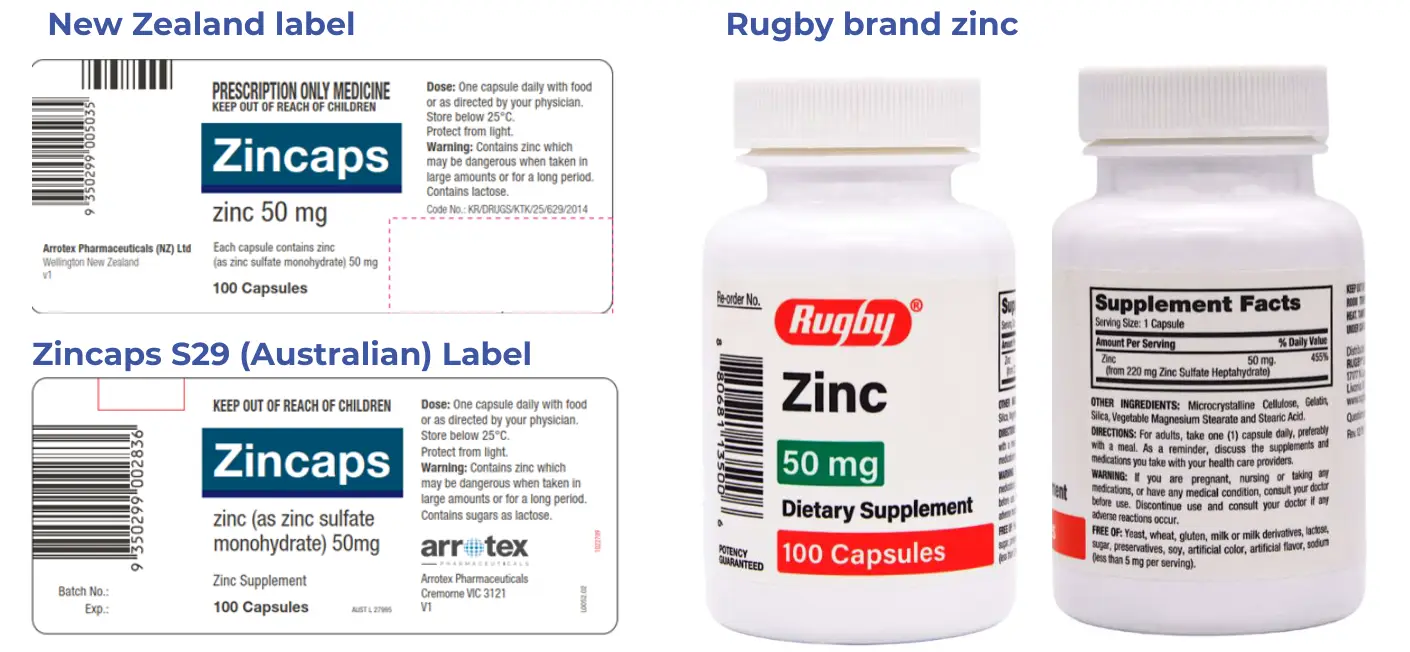
Zincaps S29
We listed this alternative from 1 October 2025. This product is exactly the same as the NZ-registered Zincaps but is packaged for Australia. The key difference is that the New Zealand label states that the medicine is prescription only.
- Chemical: Zinc sulphate
- Brand: Zincaps
- Presentation: Cap 137.4 mg (50 mg elemental)
- Pharmacode: 2714019
- Subsidy: $11.00
- Measure / Qty: per 100
- Unapproved medicine (Section 29) and wastage applies

- Zincaps supply issue | Rugby brand flier [PDF 246 KB]
- Zincaps supply issue | Zincaps s29 Flyer [PDF 255 KB]
Please keep Zincaps S29 and Rugby Zinc in dispensary
The alternative medicines do not have the ‘Prescription Medicine’ classification statement, because they are supplied for general sale in their home markets.
Please ensure that the Australian Zincaps and Rugby Zinc are kept in the dispensary and dispensed as a prescription-only medicine.
Prescribing and supplying an unapproved medicine
Section 29A of the Medicines Act 1981 allows for medicines that are not Medsafe approved to be prescribed and supplied to people.
We know supplying an unapproved medicine. In this case, however, this will allow people to continue to access an appropriate treatment.
We apologise for any inconvenience this causes. (external link)
Prescriber and pharmacist requirements for section 29 medicines – Medsafe website(external link)
What patients need to know about unapproved medicines – Healthify website(external link)
Expected resupply April 2026
The supplier, Arrotex, expects the Medsafe-registered product will be available again in April 2026.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


