Dermatological extemporaneously compounded products and galenicals
Dermatological ECPs will be funded if there is one or more subsidised dermatological galenical(s) in a subsidised dermatological base.
Dermatological bases can also be used to dilute proprietary Topical Corticosteroid-Plain preparations.
Part 7.5 of the Schedule Rules covers dermatological preparations
Dermatological bases
The products at the following links are all dermatological bases
Barrier creams and emollients(external link)
Topical corticosteroids - plain(external link) (except hydrocortisone powder)
Collodion flexible(external link) (Colloidon flexible has been discontinued by the manufacturer, it will be delisted in the future).
Dermatological galenicals
Dermatological galenicals are only subsidised when added to a dermatological base. More than one dermatological galenical can be added to a dermatological base.
- Coal tar solution BP - up to 10%(external link)
- Hydrocortisone powder - up to 5%(external link) *
- Salicylic acid powder(external link)
- Sulphur precipitated powder(external link)
- Menthol crystals(external link)
* Hydrocortisone powder is subsidised only in combination with a barrier cream/emollient, not with a proprietary topical corticosteroid-plain.
Is it funded?
For an ECP to be subsidised, it must contain 2 or more subsidised component pharmaceuticals listed in the Schedule.
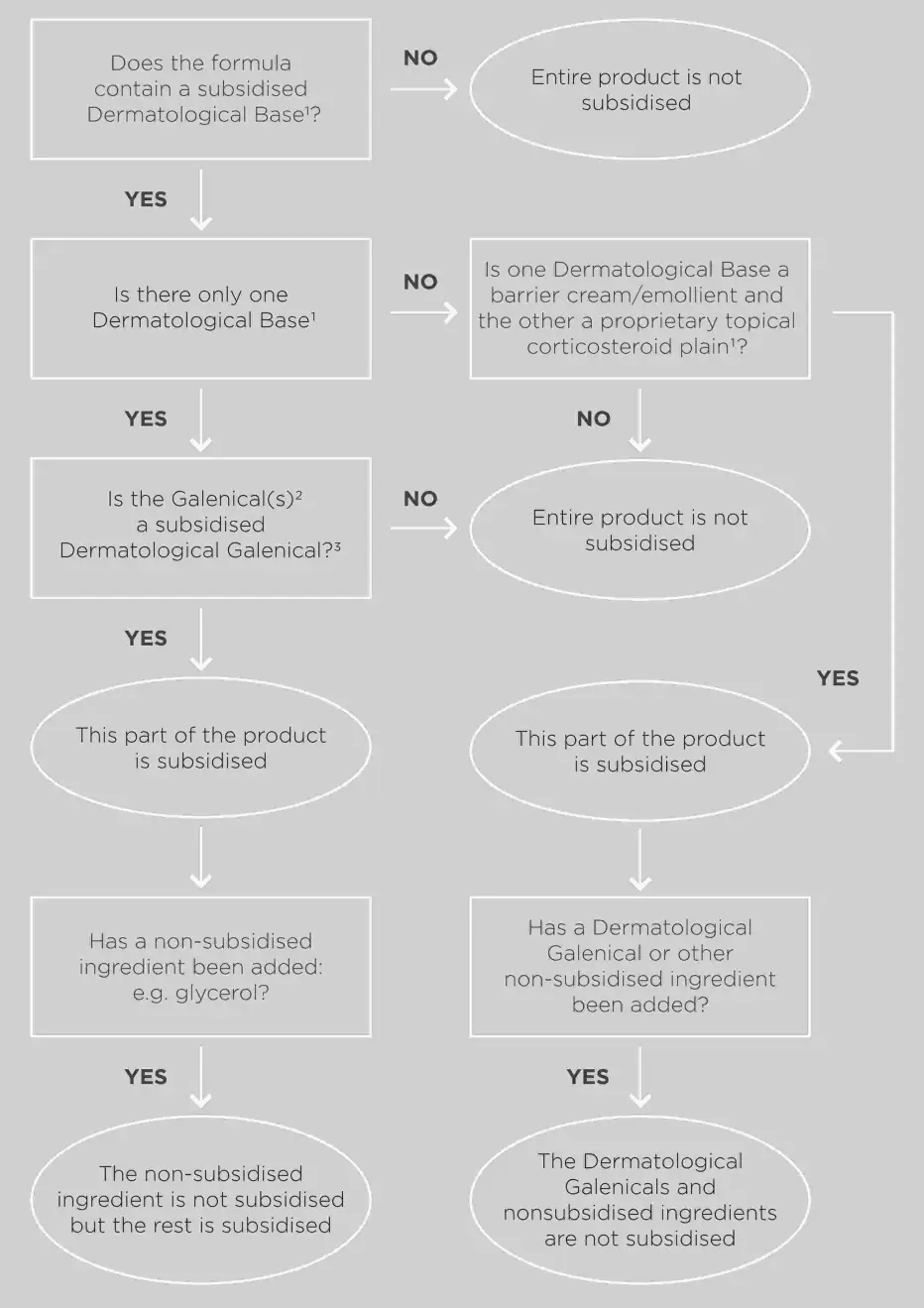
- Dermatological ECP Flowchart (2020) [PDF 367 KB]
Notes:
* Hydrocortisone powder is subsidised only in combination with a barrier cream/emollient, not with a proprietary topical corticosteroid-plain.
Information on this page is guidance only
You are responsible for making sure you comply with the lastest Schedule, including the rules in Section A.
Who to contact
If you have any questions, email enquiry@pharmac.govt.nz


