Ethosuximide (Zarontin) 250 mg capsules: Brand change
The name of your ethosuximide is changing to Zarontin.
13 August 2025 | New Zarontin available in August
The new Zarontin has arrived and will start being supplied in August 2025.
What's happening?
The supplier of Ethosuximide Essential Generics is changing the brand name for your ethosuximide to Zarontin. The packaging will look different but everything else is staying the same as the medicine you are currently taking.
It's important to keep taking ethosuximide
Suddenly stopping epilepsy medicines can cause an increase in seizures. Your doctor, nurse, or pharmacist will talk with you and answer your questions about this medicine change.

The new brand looks and works the same way
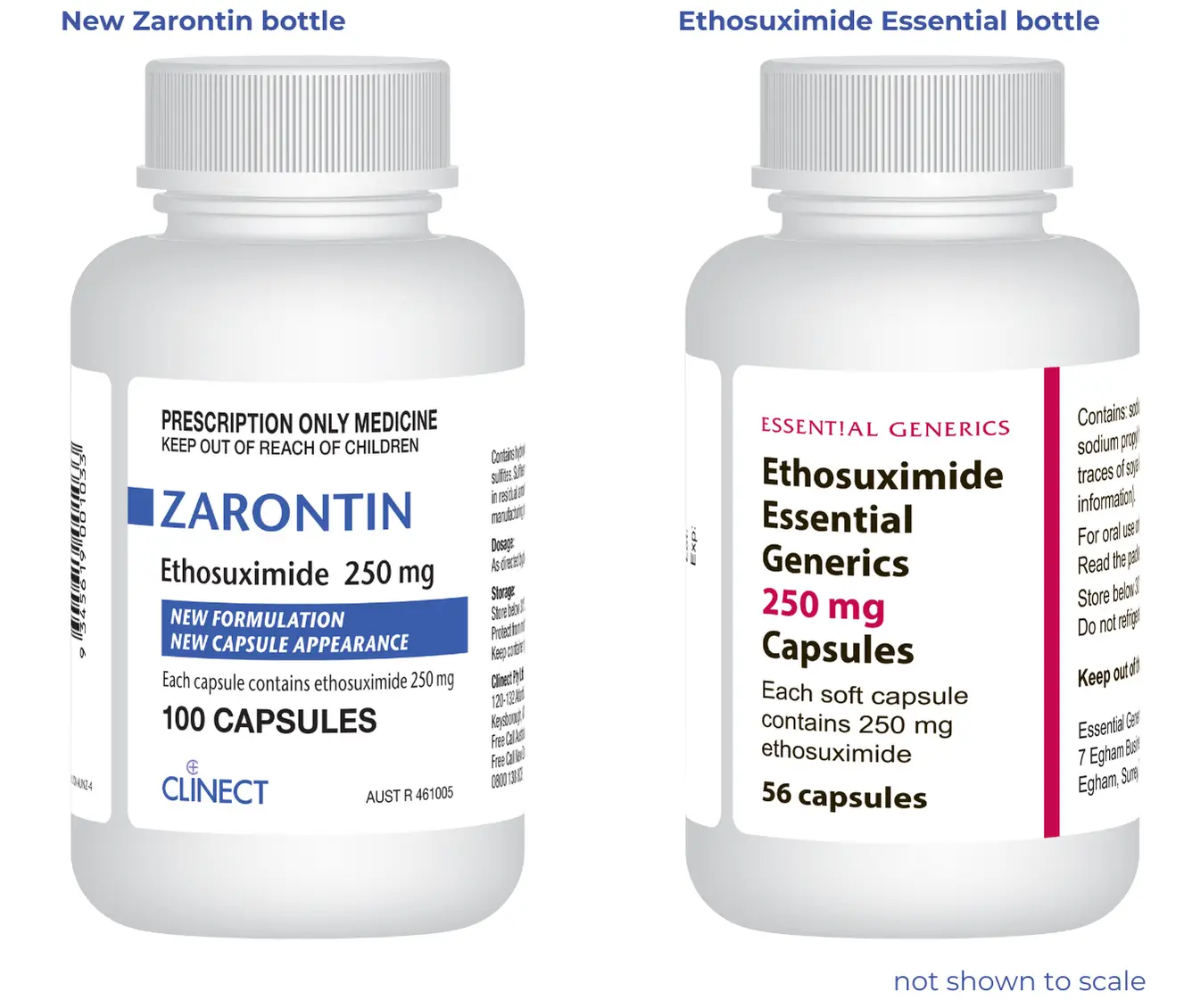
The Zarontin packaging will look different, but the capsules will look the same. Each capsule has the same formulation as your current Essential Ethosuximide. It will work in your body in the same way.
Zarontin is made by the same manufacturer as Essential Ethosuximide.
You may be familiar with the name Zarontin. This was the brand supplied before Essential Ethosuximide. The old Zarontin brand had orange capsules.
Zarontin is Medsafe approved
Medsafe has reviewed Zarontin ethosuximide and established that it meets standards of safety, quality, and effectiveness.
Medsafe is the government agency responsible for medicine safety in Aotearoa New Zealand.
Datasheet for Zarontin | Medsafe website [PDF](external link)
Consumer medicine information sheet for Zarontin | Medsafe website [PDF](external link)
New label for Zarontin
Zarontin brand ethosuximide will have a new label. The label says "new formulation, new capsule appearance" because the new Zarontin (clear capsules) is different from the old Zarontin (orange capsules).
Information for pharmacists
Clinect will replace Essential Ethosuximide 250mg capsules with Zarontin 250mg capsules. This is a return to the original brand of this medicine.
The new Pharmacode (2705117) is listed from 1 June 2025. Zarontin will come onto the market in August 2025. The Essential Ethosuximide brand will remain listed until 1 December 2025 to allow pharmacists time to claim.
Schedule listing for ethosuximide(external link)
What is changing?
- The new name is Zarontin
- The packaging is different and is a 100 pack (Essential Ethosuximide is a 56-pack)
- Zarontin capsules are Medsafe approved (Essential Ethosuximide is unapproved)
- New pharmacode 2705117 listed 1 June 2025
What remains the same?
- The supplier is still Clinect
- The Zarontin capsules are the same formulation as the Essential Ethosuximide 250mg capsules
- The Zarontin capsules are identical to the clear capsules - Essential Ethosuximide 250mg capsules that have been available since late 2023
Patients can be reassured that they will taking the same medicine that has been available since late 2023, but there will be a change to the brand name.
- Ethosuximide brand change to Zarontin A5 Flier [PDF 170 KB]
Key dates
1 June 2025
New Zarontin listed (Pharmacode: 2705117)
Old Zarontin de-listed (Pharmacode: 2489937)
August 2025
New Zarontin will start to be supplied
1 December 2025
Essential Ethosuximide de-listed (Pharmacode: 2641690)
Who to contact?
If you or a member of your whānau take this medicine, talk with your doctor, nurse, or pharmacist if you have any concerns.
You can also get more information from us at enquiry@pharmac.govt.nz
Useful links
Information for consumers - Healthify(external link)
Ethosuximide NZ Formulary monograph(external link)
Medsafe datasheet for Zarontin brand [PDF](external link)
Medsafe patient information for Zarontin [PDF](external link)


