Quetiapine (Quetapel) 25 mg tablet: Supply issue resolved
A delivery of quetiapine 25 mg tablets (Quetapel | Pharmacode: 2476266) was delayed.
17 July 2025 | Resupplied
Stock of Quetapel has arrived and been distributed to wholesalers. It can take another 1 to 2 weeks to reach pharmacies around the motu.
Affected product
Because of a delay, the supplier may go out of stock of the following product in July 2025.
- Chemical: Quetiapine
- Presentation: Tab 25 mg
- Brand: Quetapel
- Pharmacode: 2476266
- Subsidy: $2.36
- Measure / Qty: per 90
Other strengths of quetiapine remain unaffected by this supply issue.
Schedule listing for quetiapine(external link)
Alternative product available
Two alternative pack sizes were listed from 1 April 2025 for an earlier issue.
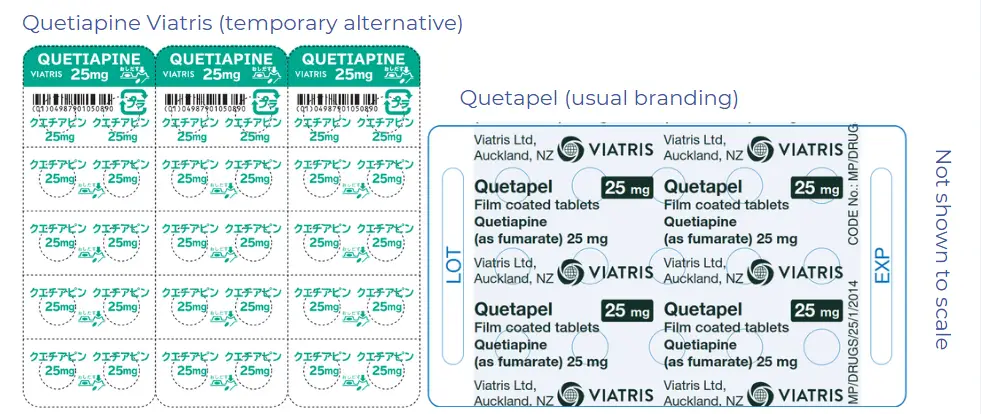
Same medicine, different look
This quetiapine has the same active ingredients in the same amount. However, the tablets and blister packs will look different.
The blister packs are labelled for people in Japan. Viatris has re-labelled the outside box for New Zealand, but the blister packs have Japanese writing on them.
Brand: Quetiapine Viatris
Presentation: Tab 25 mg
- Pharmacode: 2701162
- Subsidy: $0.79
- Measure / Qty: per 30
- wastage applies
- Pharmacode: 2701170
- Subsidy: $13.11
- Measure / Qty: per 500
- Wastage applies
Please do not cut the foil strip, this ensures the quetiapine wording remains visible.
- Quetiapine supply issue flyer Apr 2025 [PDF 683 KB]
Prescribing and supplying an unapproved medicine
Section 29 of the Medicines Act 1981 allows for medicines that are not Medsafe approved to be prescribed and supplied to people. The medicine must be prescribed by someone registered with the Medical Council of New Zealand – such as, a doctor.
We know supplying a medicine under section 29 is not ideal. In this case, however, this will allow patients to be able to access an appropriate treatment.
We apologise for any inconvenience this causes.
Advice for prescribing under section 29 – BPAC website(external link)
Prescriber and pharmacist requirements for section 29 medicines – Medsafe website(external link)
Medsafe’s section 29 Declaration / Notification Form [DOC](external link)
What patients need to know about unapproved medicines – Healthify website(external link)
Resupplied mid-July 2025
Stock has arrived and been distributed to wholesalers. It can take another 1 to 2 weeks to reach pharmacies around the motu.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, Pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


