Nortriptyline: Your brand is changing from Norpress to Allegron
The supplier of Norpress tablets is no longer supplying them to New Zealand. Allegron is replacing the Norpress brand.
New brand, same medicine
The supplier of Norpress has decided to stop supplying these tablets to New Zealand Aotearoa. We have found a replacement brand, Allegron.
Allegron has the same active ingredient in the same amount. It is designed to work in your body the same way. Allegron has been approved by Medsafe.
Medsafe is the government agency responsible for making sure medicines meet New Zealand standards of safety, quality and effectiveness.
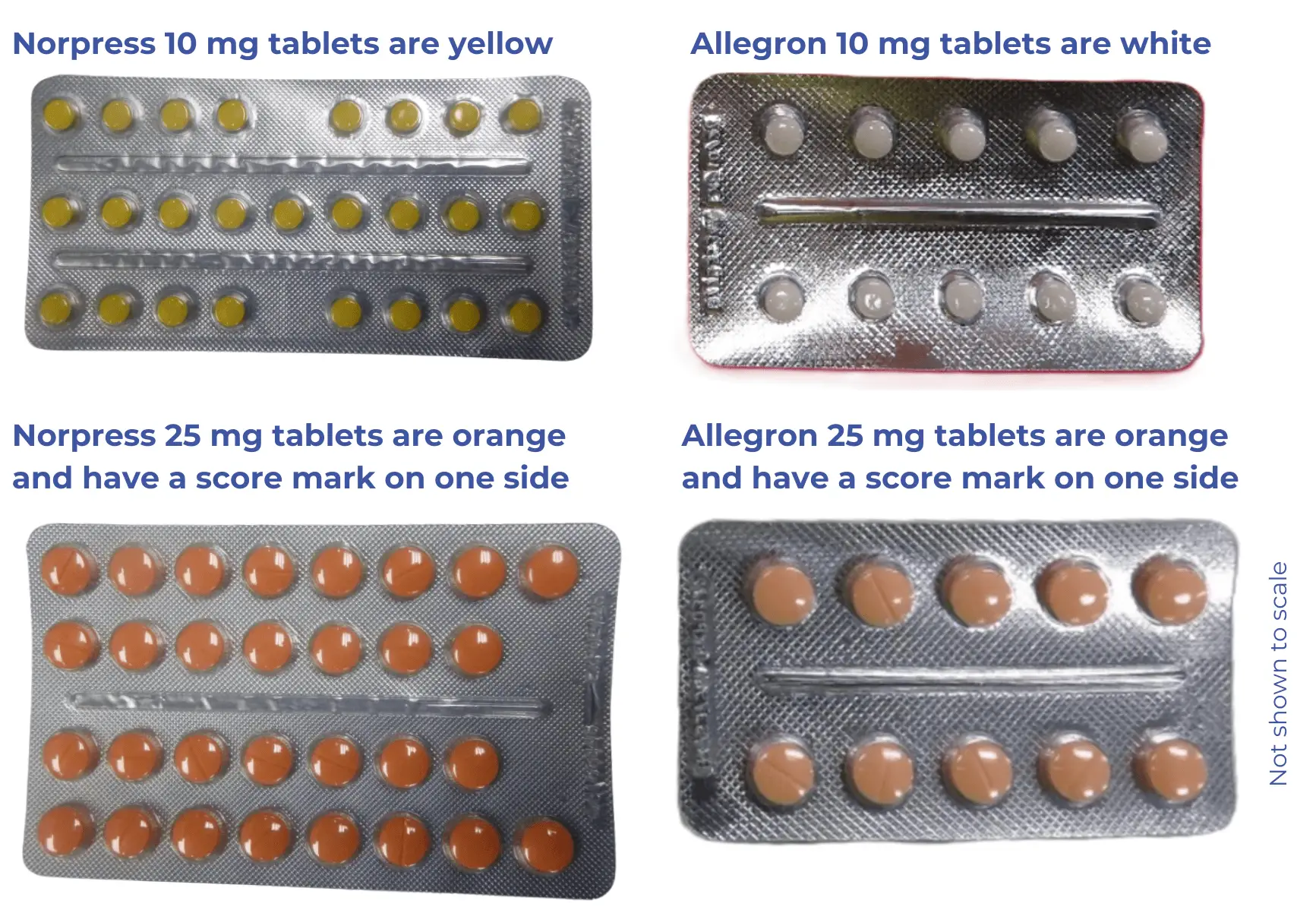
What's changing?
While the active ingredient is the same, some of the other ingredients have changed. The tablets and packaging will also look different.
Keep taking your nortriptyline
Stopping this medicine suddenly may make you feel unwell. If you do want to stop taking it, seek the guidance of your doctor. You need to slowly reduce the dose of this medicine before stopping it completely.
If you notice any changes to your symptoms, your prescriber or pharmacist are the best people to talk to.
Pharmacists
Thank you for supporting this brand change. Unfortunately, given the supplier's decision to withdraw this product from the New Zealand market, there is a short window of time to change people over to the new brand.
We have developed a flyer to support discussions with affected people.
- Nortriptyline brand change A5 flier | August 2025 [PDF 1.9 MB]
Affected products
Note the pack size for Allegron is smaller than Norpress.
| Brand | Presentation | Pharmacode | Subsidy | Quantity/per |
|---|---|---|---|---|
| Norpress | 10 mg tab | 2090678 | $2.46 | per 100 |
| Norpress | 25 mg tab | 2312441 | $6.29 | per 180 |
| Allegron | 10 mg tab | 2710692 | $2.24 | per 50 |
| Allegron | 25 mg tab | 2710706 | $2.95 | per 50 |
More information
Allegron data sheet | Medsafe [PDF](external link)
Allegron Consumer information sheet | Medsafe [PDF](external link)
Norpress data sheet | Medsafe [PDF](external link)
Norpress consumer information sheet | Medsafe [PDF](external link)
Key dates
1 August 2025 | Allegron tablets listed on the Schedule
December 2025 | Stocks of Norpress tablets likely to start running out
1 March 2026 | Norpress tablets will be delisted from the Schedule and no longer funded
Who to contact
If you have concerns about this change, please talk to the person who prescribes your nortriptyline.
Stopping this medicine suddenly may make you feel unwell. The dose of this medicine should be reduced slowly (under the guidance of your doctor) before stopping it completely.
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)
Pharmac is a government agency. We do not sell medicines or related products.


