Lanreotide (Mytolac) 60 mg per 0.5 ml syringe
There is a supply issue affecting the 60 mg strength of lanreotide injections (Pharmacode 2698145).
25 November 2025 | About the alternative
The outer packaging for the alternative 60 mg strength lanreotide injection is in English. The injection and foil pack inside are labelled in Italian, with the brand name Myrelez.
It has the same active ingredient in the same strength as the Mytolac brand.
Affected product
The supplier, Boucher & Muir, has run out of the following strength of lanreotide:
Presentation: inj 60 mg per 0.5 ml, 0.5 ml syringe
- Brand: Mytolac
- Pharmacode: 2698145
- Subsidy: $382.77
- Measure / Qty: per 1
Schedule listing for lanreotide(external link)
HML listing for lanreotide(external link)
People who use lanreotide
You may need to talk to the person who prescribed your lanreotide about the best way to respond to this supply issue.
A different brand of the 60 mg lanreotide is available. It contains the same active ingredient in the same amount. The outer packaging is in English but the foil packaging and injection inside are labelled in Italian with the brand name, Myrelez.
It has not been approved by Medsafe for use in New Zealand, this means your doctor and pharmacist have extra steps they need to follow before giving you the lanreotide.
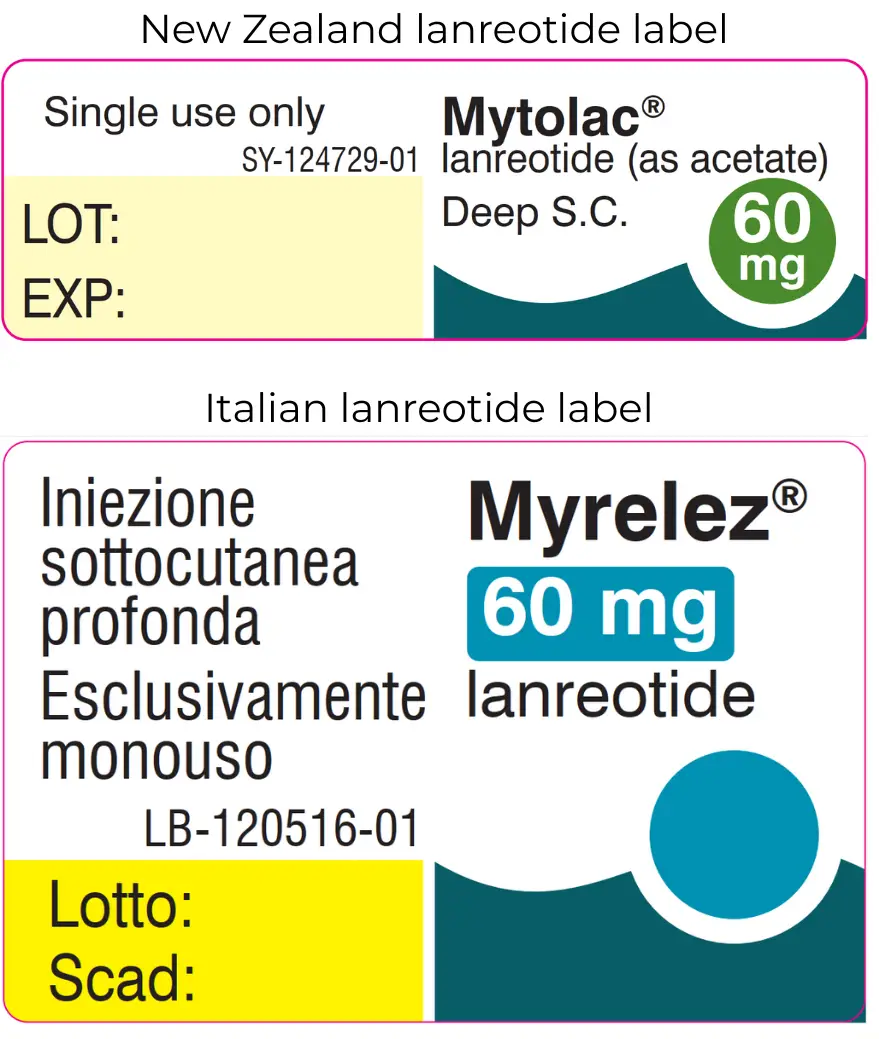
Alternative 60 mg strength
The supplier has found an alternative product for this strength. It comes with a New Zealand labelled outer box but the label on the injection is in Italian with the brand name Myrelez. The image shows both the New Zealand and Italian labels side by side.
It was listed from 1 July 2025 and is available. It is not Medsafe approved. Which means it will need to be prescribed and dispensed in line with section 29 of the Medicines Act.
- Presentation: inj 60 mg per 0.5 ml, 0.5 ml syringe
- Brand: Mytolac (s29)
- Pharmacode: 2709597
- Subsidy: $382.77
- Measure / Qty: per 1
Prescribing and supplying an unapproved medicine
Section 29 of the Medicines Act 1981 allows for medicines that are not Medsafe approved to be prescribed and supplied to people. The medicine must be prescribed by someone registered with the Medical Council of New Zealand – such as, a doctor.
We know supplying a medicine under section 29 is not ideal. In this case, however, this will allow patients to be able to access an appropriate treatment.
We apologise for any inconvenience this causes.
Advice for prescribing under section 29 – BPAC website(external link)
Prescriber and pharmacist requirements for section 29 medicines – Medsafe website(external link)
Medsafe’s section 29 Declaration / Notification Form [DOC](external link)
What patients need to know about unapproved medicines – Healthify website(external link)
Flyer for pharmacists
- Unapproved Medicine flier A5 [PDF 58 KB]
Expected resupply
The supplier expects to have registered stock of the 60 mg Mytolac available again in 2026.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


