Phytomenadione (Konakion MM) Inj 2 mg per 0.2 ml: Supply issue resolved
There has been a supply issue affecting Konakion MM (Inj 2 mg per 0.2 ml) Pharmacode 911828.
13 November 2025 Update
The New Zealand-labelled stock is being distributed again.
Affected product
This product is mostly used in hospitals, however some stock is used in the community.
- Chemical: Phytomenadione
- Presentation: Inj 2 mg per 0.2 ml - Up to 5 inj
- Brand: Konakion MM
- Pharmacode: 911828
- Subsidy: $8.00
- Measure / Qty: per 5
The Inj 10 mg per ml, 1 ml presentation (Pharmacode 298204) is unaffected by this issue.
Schedule listing for phytomenadione(external link)
HML listing for phytomenadione(external link)
Alternative product listed 1 May 2025
We listed an alternative from 1 May 2025. This product is made by the same manufacturer, however it is labelled for a different country. The strength and dosage remains the same. There is an insert included in the box, please disregard the information on dosage included in the box and refer to the Medsafe Datasheet for Konakion [PDF](external link)
- Chemical: Phytomenadione
- Presentation: Inj 2 mg per 0.2 ml
- Brand: Konakion MM
- Pharmacode: 2703572
This product has a labelling exemption from Medsafe. It can be prescribed, dispensed and given as a registered NZ medicine. The supplier is including a letter outlining the differences in packaging with every pack of the alternative Konakion to health care professionals.
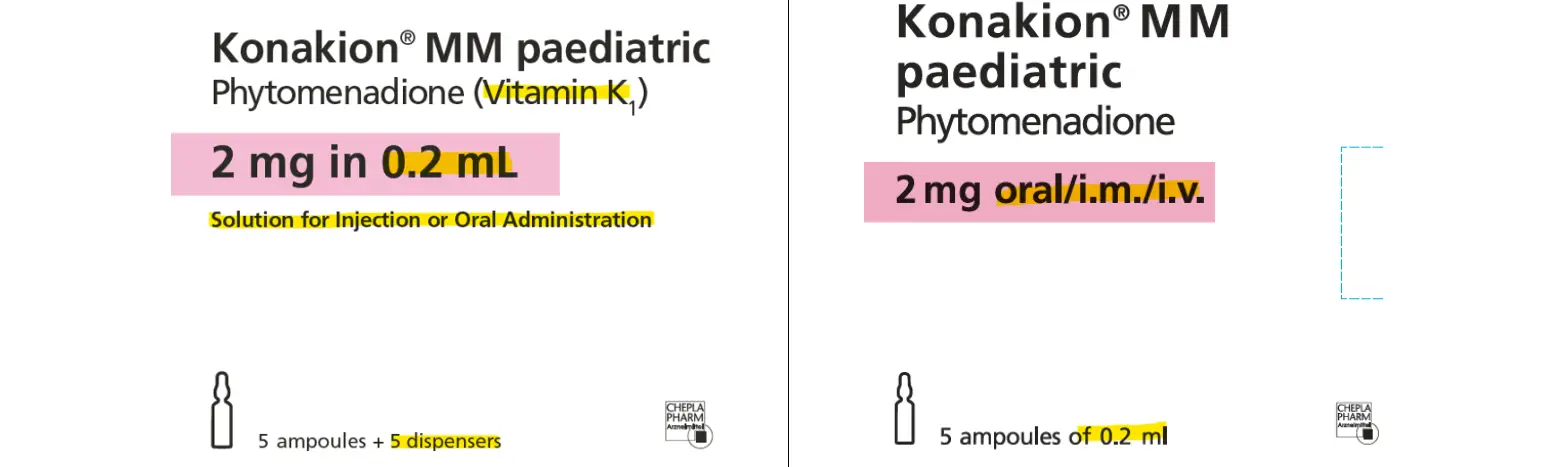
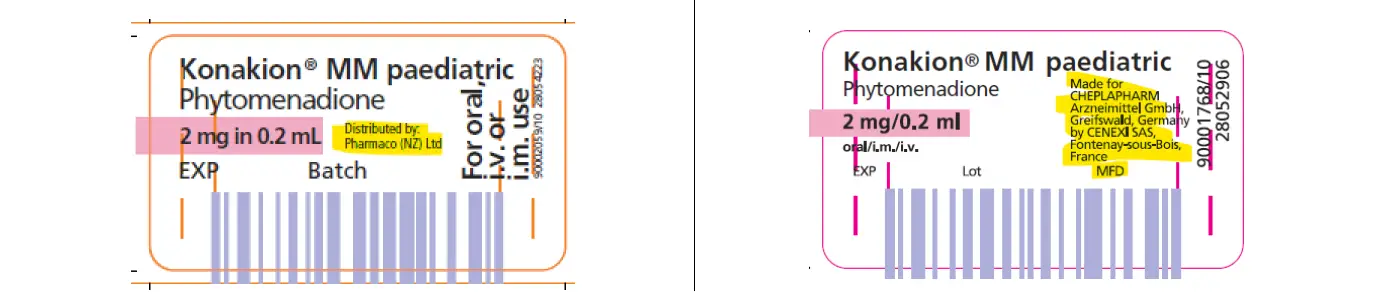
Resupplied November 2025
The New Zealand-labelled stock is being distributed again.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


