Famotidine (20 and 40 mg): New look
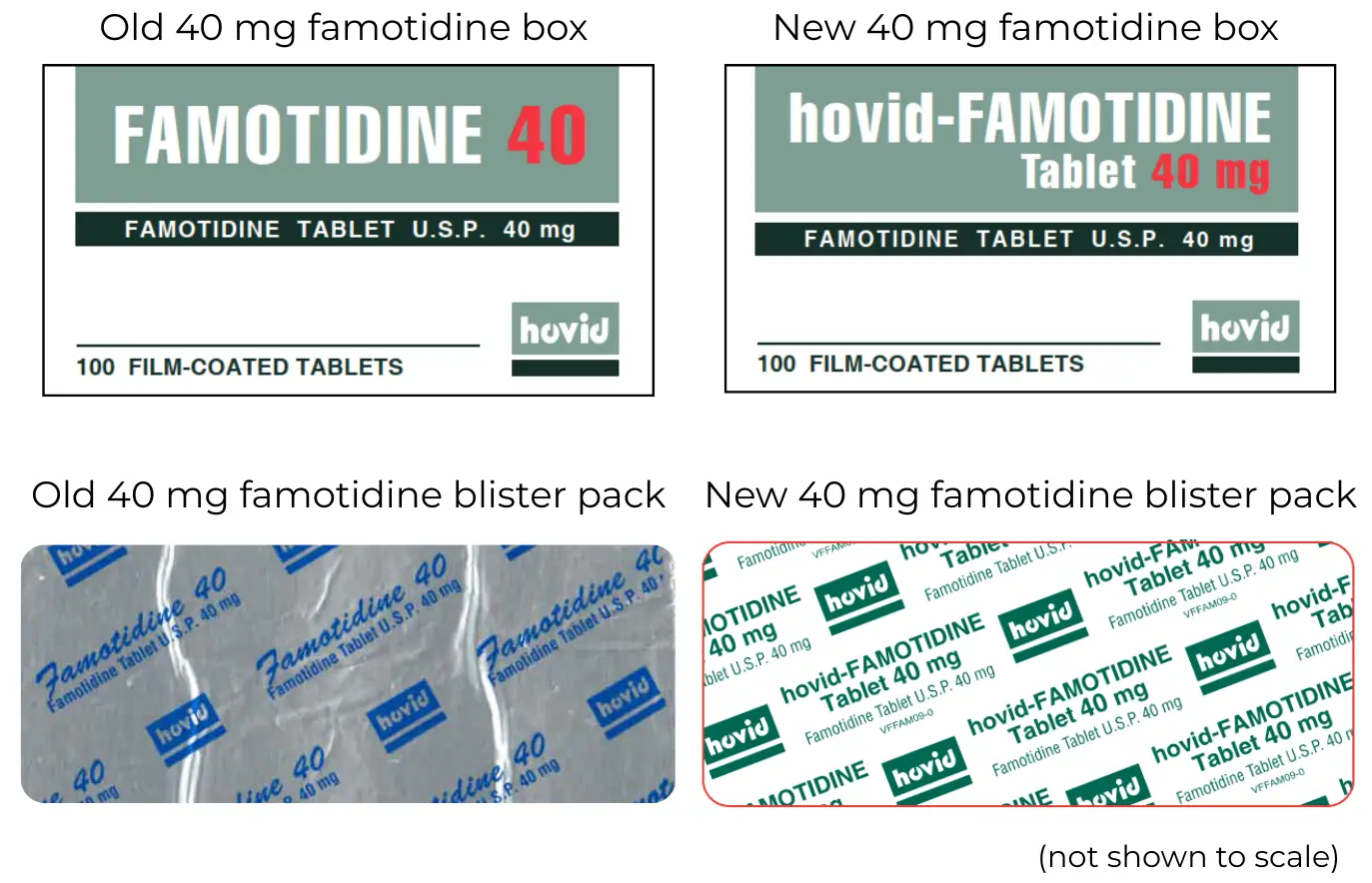
The supplier of famotidine tablets is changing. The manufacturer remains Hovid. The 40 mg tablets will have new packaging.
The supplier of famotidine is changing
The medicine being supplied is still made by the same manufacturer, Hovid. There is no change for the 20 mg tablets. The 40 mg tablets will have a new look box and blister pack.
Your famotidine contains the same amount of active ingredient and the same inactive ingredients.
Information for pharmacists
40 mg tablets new Pharmacode, new subsidy
The 40 mg tablets have a new Pharmacode which was listed from 1 June 2025.
| 40 mg tablets | Old | New |
|---|---|---|
| Pharmacode | 2581957 | 2706229 |
| Subsidy | $10.32 | $10.27 |
20 mg tablets same Pharmacode new subsidy
The only difference for the 20 mg tablets is a new lower subsidy from 1 September 2025.
|
20 mg tablets |
Old |
New |
|---|---|---|
|
Subsidy |
$4.91 |
$4.86 |
Both presentations are still unapproved
The old Hovid famotidine was not Medsafe approved. At this stage, the new Hovid famotidine will also need to be supplied as an unapproved medicine. However, the new supplier, AFT, plans to register this product with Medsafe.
Flier to support conversations with patients
- Famotidine change 2025 A5 Flier [PDF 355 KB]
Key dates
1 June 2025
- New Pharmacode and subsidy listed for the 40 mg tablet
1 September 2025
- 20 mg tablet: new subsidy listed
- 40 mg tablet: old Pharmacode delisted
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, Pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)


