Fulvestrant: Your brand is changing
The funded brand of fulvestrant is changing from Faslodex to Fulvestrant EVER Pharma.
On this page
Your new brand is Fulvestrant EVER Pharma
Between 1 December 2025 and 1 May 2026, your brand of fulvestrant will change from Faslodex to Fulvestrant EVER Pharma. We understand that changing brands can raise questions. We've tried to answer as many as we can here.
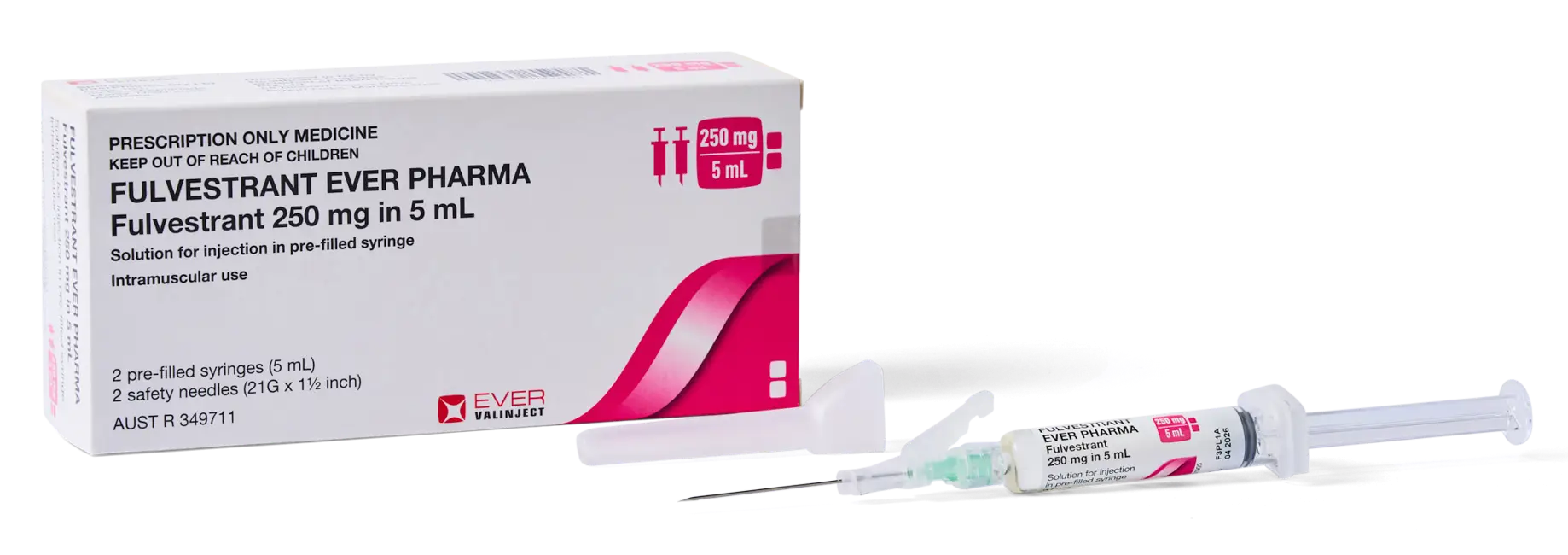

Same active ingredient, same effectiveness
Fulvestrant EVER Pharma has the same active ingredient, in the same quantity, and will work in your body the same way as Faslodex. Medsafe has approved Fulvestrant EVER Pharma for use in New Zealand. Medsafe confirms that it meets New Zealand standards of safety, quality, and effectiveness.
Already used in Australia
The Fulvestrant EVER Pharma brand is already widely used in Australia without identifiable concerns. The New Zealand Fulvestrant EVER Pharma is made in the same factory, using the same process as the Australian product.
Store at room temperature
Unlike Faslodex, Fulvestrant EVER Pharma should be stored at room temperature (under 25 degrees). This means it doesn't need to be warmed up before administration – which can help reduce pain at the injection site.
More secure supply lines
Fulvestrant EVER Pharma is also supplied in Australia and doesn't need to be refrigerated. This means things are less likely to wrong (no refrigerated transport needed) but if they do, it's easier to get extra stock to New Zealand.
What happens if I have a bad reaction to the new brand?
We are confident that most people will respond the same to the Fulvestrant EVER Pharma brand as they do the Faslodex brand. However, everyone is different. If your prescriber finds that Fulvestrant EVER Pharma is not clinically suitable, you may be able to access a different brand through a special Pharmac funding pathway, called Named Patient Pharmaceutical Assessment (NPPA).
Finding more information
We recommend you talk the person who prescribes your fulvestrant and to your pharmacist. They can discuss what this brand change might mean for you, what your treatment options are, and how to manage any side effects.
You can find more information at Healthify's website(external link)
Health professionals: What you need to know
Thank you for all the support you give patients through brand changes. We know that brand changes for breast cancer can raise concerns for people taking the medicines. We appreciate the time you take to support people through these changes.
We did not take this change lightly; we sought advice from clinical advisors throughout the process. Where possible, we use products that are already in use in comparable health systems, such as Australia and the United Kingdom, where regulatory standards and clinical expectations align closely with those in New Zealand.
About Fulvestrant EVER Pharma
Like Faslodex, Fulvestrant EVER Pharma is Medsafe approved. It is currently supplied in Australia where there have been no identifiable concerns raised with it. The New Zealand Fulvestrant EVER Pharma is clinically the same as the Australian one; it has the same formulation and it is made in the same factory.
Fulvestrant EVER Pharma can be stored at room temperature. This reduces logistical barriers and risks associated with cold chain management. In addition, it doesn't need to be warmed up before administration – this can help reduce injection site pain.
Datasheet for Fulvestrant EVER Pharma [PDF] (Medsafe)(external link)
Consumer Medicine Information for Fulvestrant EVER Pharma [PDF] (Medsafe)(external link)
Storage differences
|
Brand |
Fulvestrant EVER Pharma |
Faslodex |
|---|---|---|
|
Supplier |
InterPharma |
AstraZeneca |
|
Container type |
Prefilled syringe, 5 ml, glass, type I, fluoropolymer coated bromobutyl rubber plunger, in carton |
Prefilled syringe, 5 ml, glass, cardboard carton |
|
Pharmacode |
2663139 |
2586371 |
|
Subsidy |
|
$1068.00 |
|
Pack size |
2 |
2 |
|
Solution or suspension |
Solution for injection |
Solution for injection |
|
Colour of solution |
Clear |
Clear |
|
Shelf Life |
24 months from date of manufacture stored at or below 25°C |
4 years from date of manufacture stored at 2° to 8°C (Refrigerate, do not freeze) protect from light |
|
Excipients |
Benzyl alcohol Benzyl benzoate Castor oil Ethanol |
Benzyl alcohol Benzyl benzoate Castor oil Ethanol |
- Fulvestrant brand change flier | Nov 2025 [PDF 271 KB]
Named Patient Pharmaceutical Assessment (NPPA) pathway
If someone experiences intolerable side effects from this brand, you can consider using the NPPA pathway to seek funding for a different brand of fulvestrant.
About making a NPPA application
Key dates
1 December 2025 | Fulvestrant EVER Pharma listed in the HML and Community Schedule
1 May 2026 | Faslodex delisted from HML and Community Schedule
1 May 2026 to 30 June 2028 | Fulvestrant EVER Pharma has principal supply status in New Zealand
Why is this happening?
This is a tender brand change. Every year, Pharmac runs a tender process to create competition among suppliers to supply many medicines used by New Zealanders.
The tender helps Pharmac manage the costs of medicines. Any savings we make are returned to the medicines budget to help pay for new medicines.
This brand change is helping fund more medicines for more New Zealanders.
Consultation on this brand change
We consulted on this specific brand change as part of our recent efforts to increase consultation around brand changes caused by tender decisions.
We received significant feedback both supportive of and concerned about this brand change to fulvestrant. Thank you to everyone who gave feedback, it has helped us shape our decision.
You can read the full decision and a summary of the feedback we received and how we responded to that feedback.
Decision to brand change fulvestrant through the 2024/25 Invitation to Tender
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)
Pharmac is a government agency. We do not sell medicines or related products.


