Enalapril: Your brand is changing
Your enalapril is changing from Acetec to Ipca-Enalapril.

Same medicine with the same active ingredient
Ipca-Enalapril has the exact same active ingredient, in the same quantity, as Acetec. You should not notice any difference in how it works.
What's different?
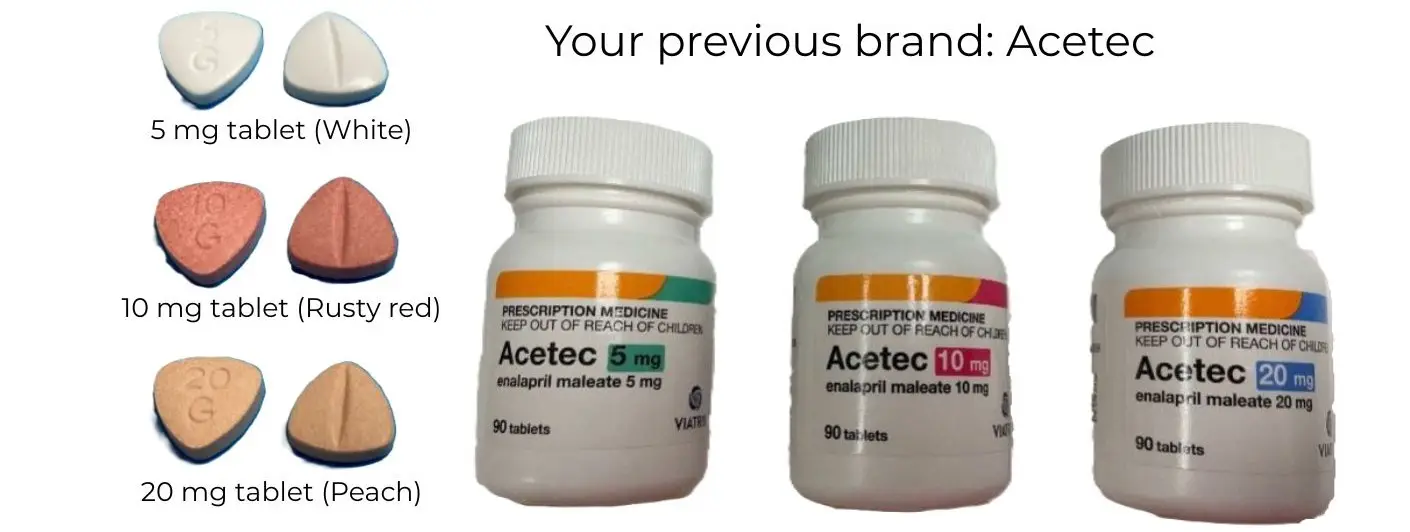
The Ipca-Enalapril comes in blister packs (rather than in a bottle). The pills are round and white. They still have a score mark down the middle and the strength, 5, 10, or 20 on them.
There are some slight differences in the inactive ingredients between Ipca-Enlapril and Acetec. You can check these on the datasheets on the Medsafe website. Talk to a health professional you trust if you have any questions about the difference.
The pills may look familiar
If you took Ethics brand enalapril, you've taken this exact medicine before. Ipca-Enalapril is the exact same tablets as the Ethics brand, just in different packaging.
Ethics was the funded brand of Enalapril between 2013 and 2021.
When is this happening
Ipca-Enalapril was funded from 1 February 2026, Acetec will stop being funded from 1 July 2026. Your pharmacist will change your brand at some point between these dates.
Why is this happening?
This is a tender brand change. Every year, Pharmac runs a tender process to create competition among suppliers to supply many medicines used by New Zealanders.
The tender helps Pharmac manage the costs of medicines. Any savings we make are returned to the medicines budget to help pay for new medicines.
Your brand change is helping fund more medicines for more New Zealanders.
Information for prescribers of enalapril
This brand change will happen between 1 February 2026 and 1 July 2026.
Miro, the supplier, has confirmed that Ipca-Enalapril is the same product as the one supplied by Multichem (Ethics) between 2013 and 2021, however, in different packaging.
Please monitor patients
Prescribers should monitor patients following any change in medication and make adjustments as required. The active ingredient for both Ipca-Enalapril and Acetec are from the same supplier. However, some of the inactive ingredients (excipients) are different.
Please clearly document any adverse experiences related to a brand switch.
CARM reporting form(external link)
Information for pharmacists
The new Pharmacodes are:
- tab 5 mg 2716771
- tab 10 mg 2716798
- tab 20 mg 2716801
Please clearly document any adverse experiences related to a brand switch.
CARM reporting form(external link)
New brand in blisters and new shape
Ipca-Enalapril comes in blister foil packs, where Acetec came in bottles. The tablets are also a different shape.
We acknowledge that these changes may affect pharmacies using robots for dispensing. We are sorry for any inconvenience caused.
Patient flyer
We've developed a flier to support conversations with people affected by this brand change. Thank you for the time and care you give through brand changes.
- Enalapril brand change flier [PDF 270 KB]
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)
Pharmac is a government agency. We do not sell medicines or related products.


