Condyline (podophyllotoxin): Supply issue resolved
There was a supply issue affecting Condyline (Pharmacode: 701610)
15 January 2026 | Condyline resupplied
Stock of the New Zealand product is available from the supplier again.
Affected product
AFT advises that its most recent shipment of Condyline can't be released because of temperature fluctuations during shipping.
- Chemical: Podophyllotoxin
- Presentation: Soln 0.5%
- Brand: Condyline
- Pharmacode: 701610
- Subsidy: $33.60
- Measure / Qty: per 3.5 ml OP
Schedule listing for Condyline(external link)
Alternative product
The supplier has found an alternative. It is exactly the same product but is labelled in French. It was listed in the Schedule from 1 October 2025 and is available to order.
Where Condyline is unavailable
Unfortunately, the recommended alternative, imiquimod cream (Pharmacode: 2543907) is also unavailable. We are seeking further clinical advice on alternatives.
People should talk to the person who prescribed the Condyline. That person will be in the best place to discuss options with you.
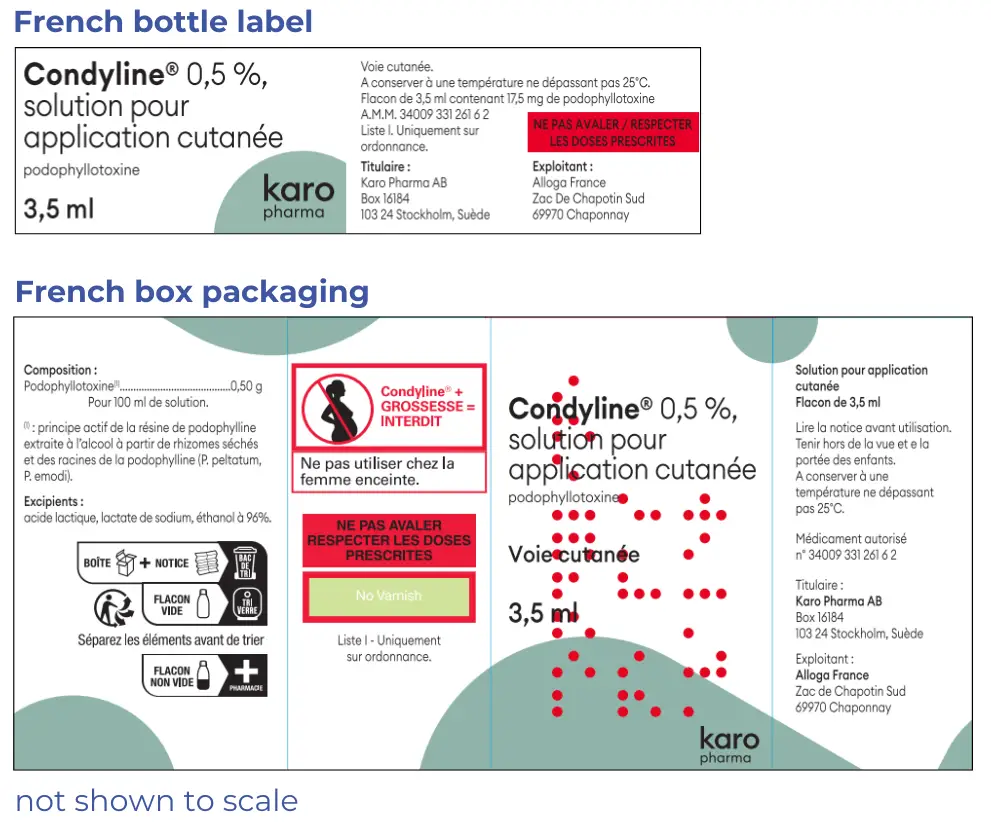
French Condyline
AFT will overlabel one panel of the box with English, but the other panels and bottle will remain in French. An English language consumer medicine information sheet will be included in the box.
Pharmacists, please consider over labelling the bottle with the pharmacy generated label.
- Chemical: Podophyllotoxin
- Presentation: Soln 0.5%
- Brand: Condyline S29
- Pharmacode: 2714205
- Subsidy: $33.60
- Measure / Qty: per 3.5 ml OP
- Section 29
The chemical name appears differently in French, podophyllotoxine, instead of podophyllotoxin.
English Patient information leaflet Medsafe website [PDF](external link)
- Condyline supply issue [PDF 312 KB]
Prescribing and supplying an unapproved medicine
Section 29 of the Medicines Act 1981 allows for medicines that are not Medsafe approved to be prescribed and supplied to people. The medicine must be prescribed by someone registered with the Medical Council of New Zealand – such as, a doctor.
We know supplying a medicine under section 29 is not ideal. In this case, however, this will allow patients to be able to access an appropriate treatment.
We apologise for any inconvenience this causes.
Advice for prescribing under section 29 – BPAC website(external link)
Prescriber and pharmacist requirements for section 29 medicines – Medsafe website (external link)
What patients need to know about unapproved medicines – Healthify website(external link)
Resupplied January 2026
The manufacturer could not begin production of the New Zealand product until December 2025. The supplier advises that the product is available to order again.
Who to contact
If you have questions about this issue, email enquiry@pharmac.govt.nz
Please include as much information as you can about the product (presentation, brand, pharmacode) and who your wholesaler is.
Sign up to our email list for regular emails about supply issues and more(external link)
Pharmac is a government agency. We do not sell medicines or related products.


